Wood Plastic Composite based on PVC (WPVC) is a material obtained by combining rigid PVC with wood flour. This material merges the chemical resistance and self-extinguishing properties of PVC with the rigidity and natural appearance of wood. However, WPVC faces a major challenge: low thermal stability caused by the interaction between PVC and wood components.
What is WPVC?
WPVC is produced by mixing rigid PVC with wood particles. This combination provides:
• High mechanical stiffness.
• Remarkable chemical resistance.
• Self-extinguishing behavior.
The critical drawback lies in the presence of hydroxyl groups and other components in wood that catalyze PVC degradation during processing.
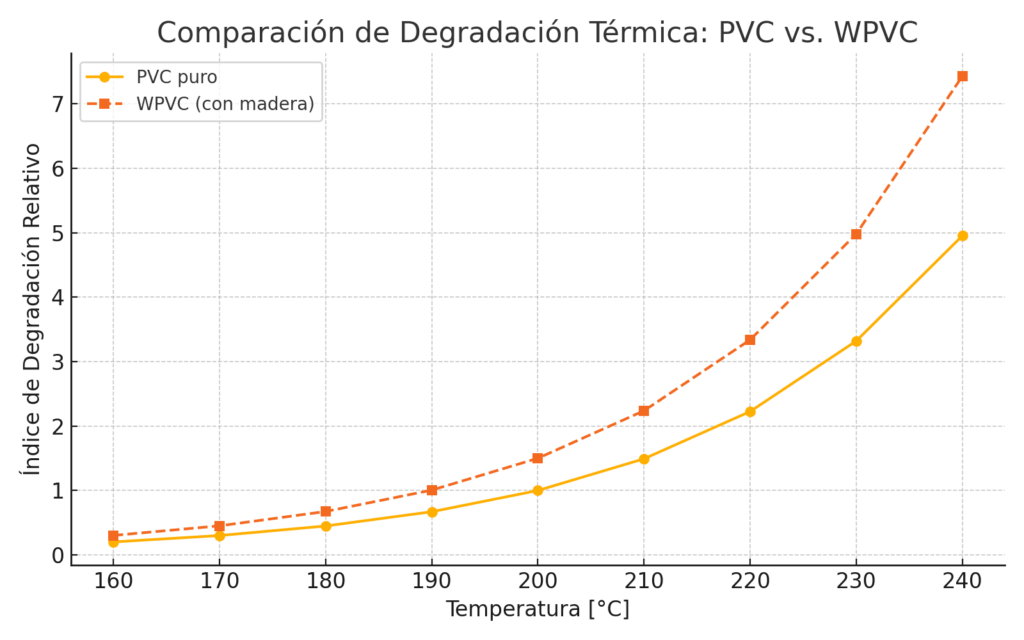
Comparison of Thermal Degradation
Thermal degradation in WPVC occurs faster than in neat PVC. This is due to the release of HCl (dehydrochlorination), which accelerates darkening, loss of mechanical strength, and reduction of service life. Therefore, adequate stabilizer systems are essential to allow proper processing and preserve compound properties.
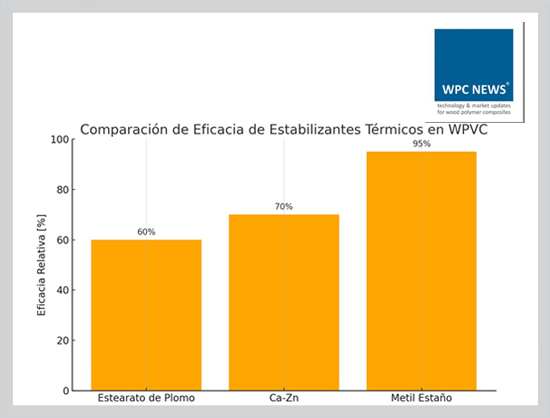
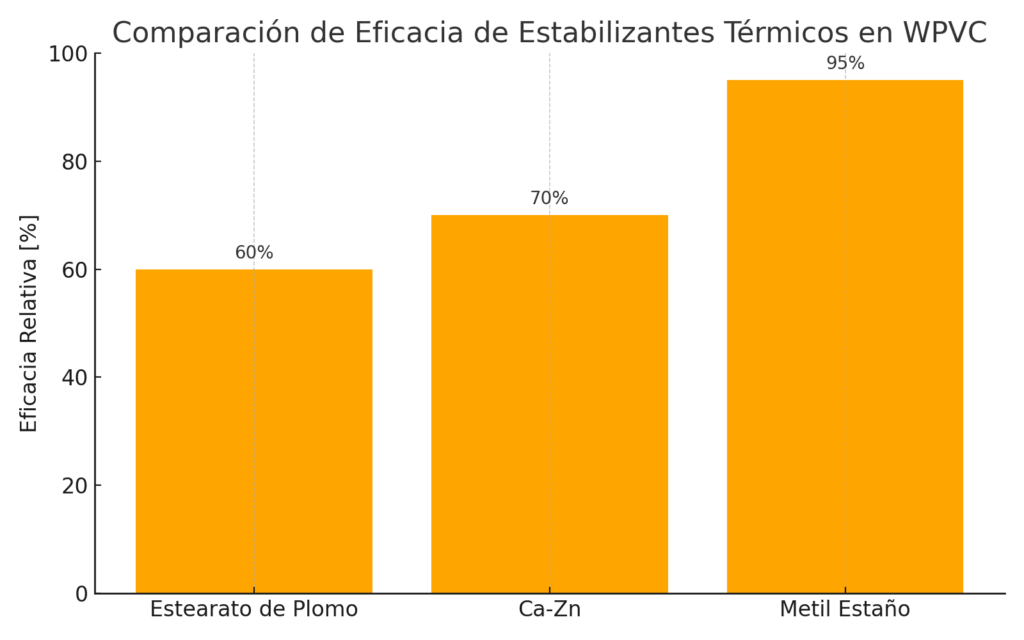
Types of Thermal Stabilizers in WPVC
Thermal stabilizers are crucial additives in WPVC. The most commonly used are:
• **Lead stearate:** Highly effective, but environmentally restricted due to toxicity concerns.
• **Calcium–zinc systems:** Heavy-metal-free alternative, offering intermediate efficiency with broader regulatory acceptance.
• **Methyl tin compounds:** Highly efficient, particularly suitable for WPVC, as they strongly suppress dehydrochlorination.
The choice depends on balancing efficiency, cost, and compliance with health and environmental regulations.
Effectiveness of Stabilizers and Color Control
The effectiveness of stabilizers is mainly evaluated through:
• Induction time before degradation.
• Retention of mechanical properties.
• Color stability during extrusion.
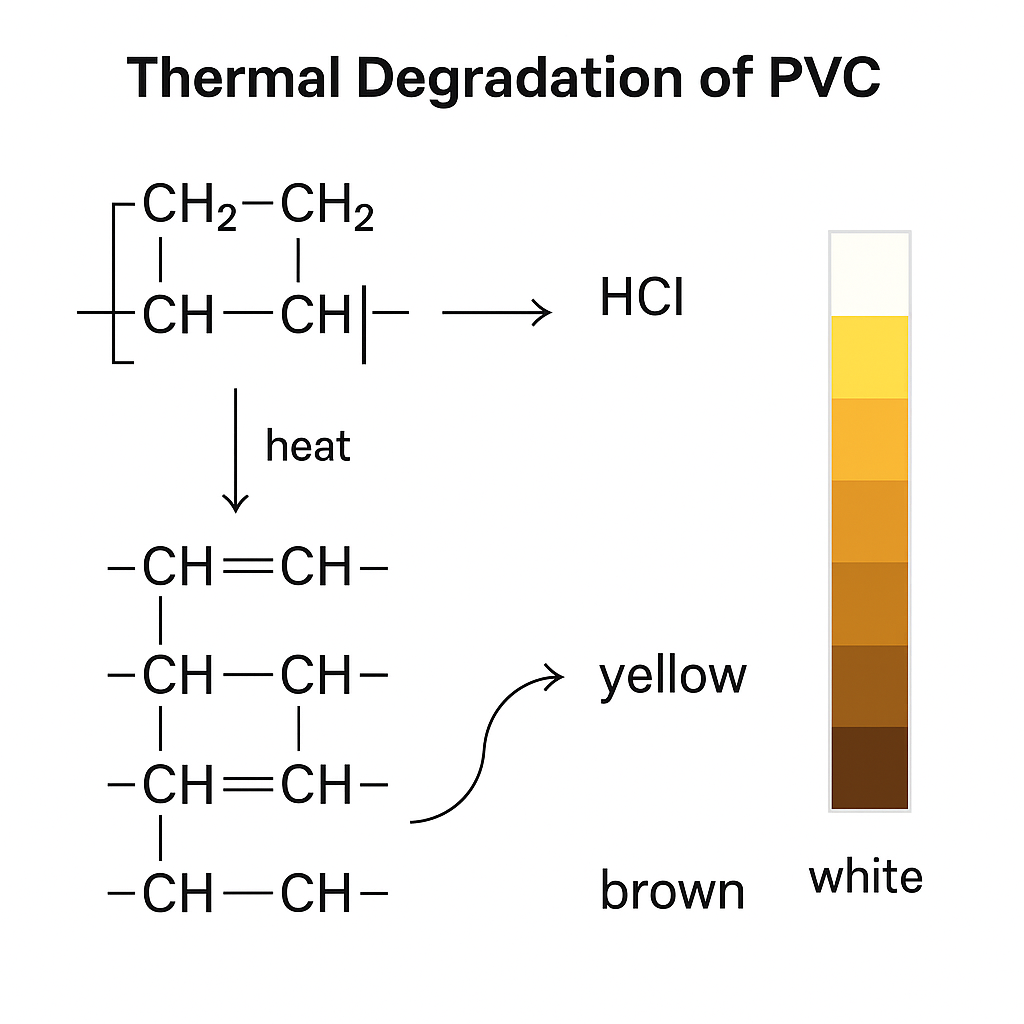
In practice, progressive discoloration is observed (yellowing to dark brown), caused by HCl release and the formation of conjugated double bonds in the PVC backbone.
Mechanism of PVC Dehydrochlorination
The primary degradation mechanism in PVC and WPVC is dehydrochlorination. This process involves HCl release, triggering a chain reaction that leads to:
• Formation of conjugated double bonds (polyene sequences).
• Progressive color change (from white to brown).
• Loss of mechanical strength.
• Shorter service life of the product.
Stabilizers act by neutralizing released HCl or by replacing labile sites along the polymer chain.
Author:
Mr Carlos Cardenas, Eng. (Argentina)
Carloscardenas1963@gmail.com
He is a Materials Engineer with more than 35 years of experience in the polymer industry, particularly in PVC and Wood-Plastic Composites (WPC). He is an independent consultant in extrusion processing of heterogeneous polymers and WPC-type composites.
References
• Sombatsompop et al. (2003). Polym. Int. 52(12), 1847–1855.
• Chaochanchaikul et al. (2011). BioResources, 6(3), 3115–3131.